
HPTLC method as a cost-saving option for quantitative evaluation of herbal drugs
By Débora Frommenwiler and Marcel Hug
A study, published in the Journal of LC&RT, compares the costs and analysis time for the evaluation of quality of Reishi mushrooms by HPTLC and a pharmacopoeial method.
Quality assessment is a fundamental step to produce safe and efficacious herbal drugs, preparations and products [1]. For quality control companies can either develop their own methods and acceptance criteria or follow pre-established methods of pharmacopoeias and other compendia [2] [3].
When adhering to compendial monographs, quality control (QC) of herbals can be a complex task, involving several steps and tests. Consequently, costs can increase rapidly. For some companies that work with low volume trade herbals, the achievable margins may not compensate the high cost of quality testing.
Having in mind the number of tests, cost and time requirements, the authors developed a case study to demonstrate that an HPTLC method can be a fast and cost-saving alternative for quality control of herbals. The paper was published in the Journal of Liquid Chromatography and Related Techniques [4]
The content of the study
For this case study, the fruiting body of Ganoderma lucidum (Curtis) P. Karst (GLFB), a Traditional Chinese Medicine (TCM) drug aka Reishi mushroom, was chosen. Currently, the United States Pharmacopoeia (USP) has a suite of tests for the QC of GLFB compiled in a monograph, which includes tests for identification by HPTLC and quantification of triterpenoic acids by UHPLC [5]. It also contains other tests that are not subject of this comparison. The authors propose a single HPTLC method for identification of GLFB, discrimination of possible confounding material, and quantification of triterpenoic acids.
Comparing the analysis cost and time of two methods
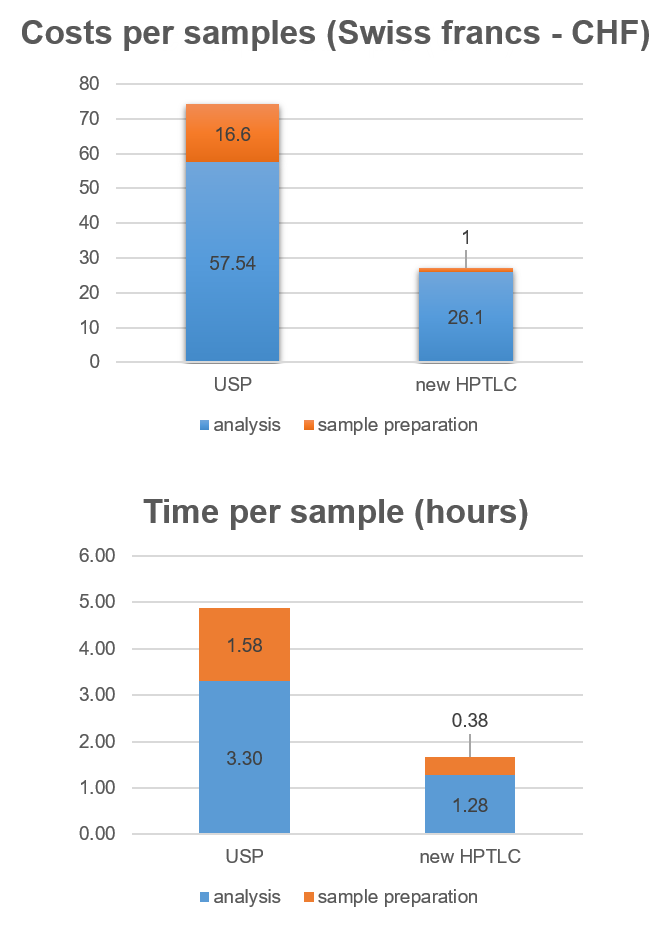
For calculating the costs and analysis time, only two steps of the QC process were considered: identification of the herbal drug and assay of markers. Within these steps, the following parameters were reflected in the calculation: volume of solvent used for the chromatography and sample preparation, costs per unit of disposable material, cost of stationary phase, and sample preparation time.
A comparison between the new HPTLC method and the combined USP method (HPTLC + UHPLC) for a single sample is shown in Figure 1. The new HPTLC method (orange bars) is more than 2.9-times faster and costs about 37% of the combined USP method (blue bars). It also uses 13-times less solvent.
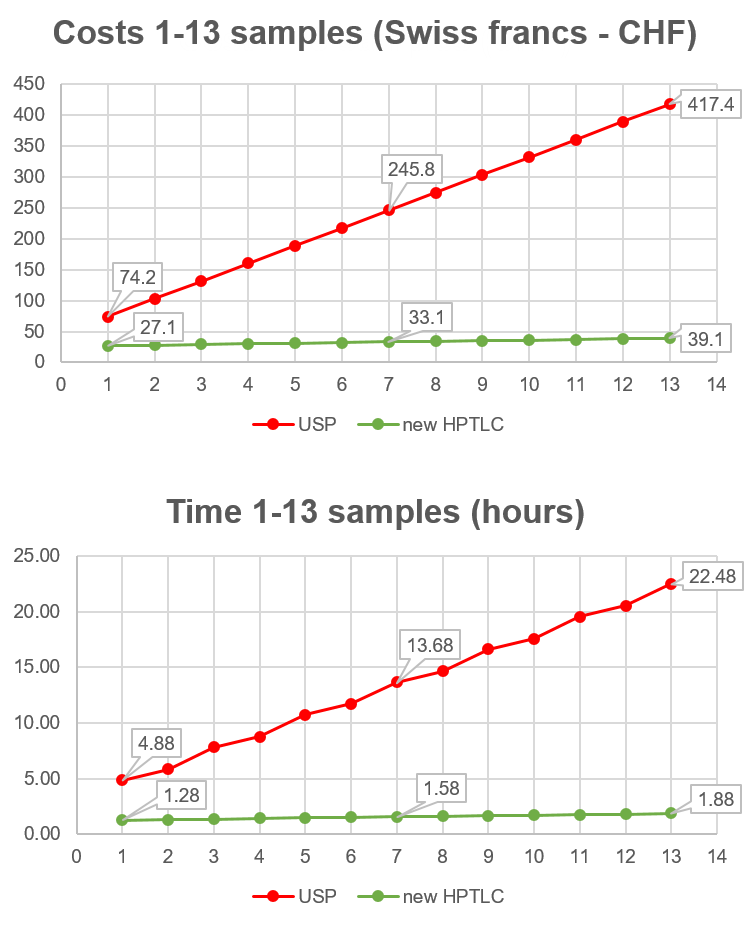
When larger numbers of samples are analyzed simultaneously, the differences in analysis time and costs between the methods increase dramatically. For example, for 13 samples, the new HPTLC method (green dots) is more than 11-times faster, costs less than 10% of the combined USP method (red dots), and uses 32-times less solvent. This is because the fixed cost of HPTLC analysis is nearly the same for the analysis of 1 or 13 samples, while UHPLC analysis is cumulative.
So, can an HPTLC method be a cost-saving alternative to existing methods?
The short answer to this question is yes, it can. As shown in this case study, the HPTLC method can help cut costs by reducing the number of tests and simplifying the QC process. In addition, it provides the same set of information as the compendial method regarding identity, purity, and content of herbal drugs.
What are the recommendations for creating a cost-saving HPTLC method?
1. Use all possible features of the HPTLC technique, e.g., perform tests for identity, purity, and content in a single HPTLC analysis
2. Simplify the sample preparation method
3. Analyze a large number of samples in a single run
[5] Monographs for Ganoderma lucidum Fruiting Body and Ganoderma lucidum Fruiting Body Powder. In The United States Pharmacopoeia (USP) 41–NF36. United States Pharmacopeial: Rockville, USA, pp 4629–4635, 2018.
Comments
Leave a comment